In Vitro Diagnostics (IVD)
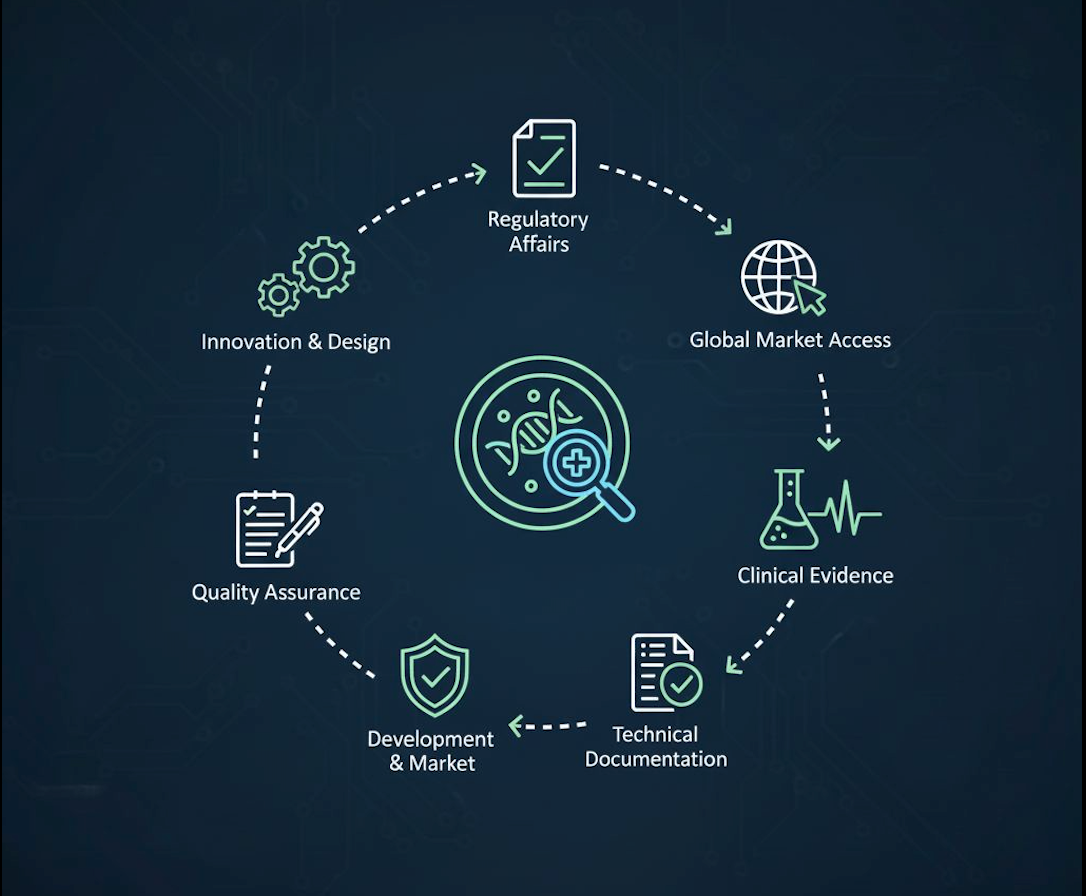
The In Vitro Diagnostics (IVD) industry is evolving rapidly, driven by technological innovations, complex regulatory landscapes, and the increasing need for global market access.
At Labmark, we help IVD manufacturers overcome these challenges with tailored solutions - from regulatory affairs and quality assurance to clinical evidence and technical documentation.
Partner with us to accelerate your journey from idea to patient.
Offering comprehensive IVD services
We provide end-to-end support across the entire IVD lifecycle. Our services include:

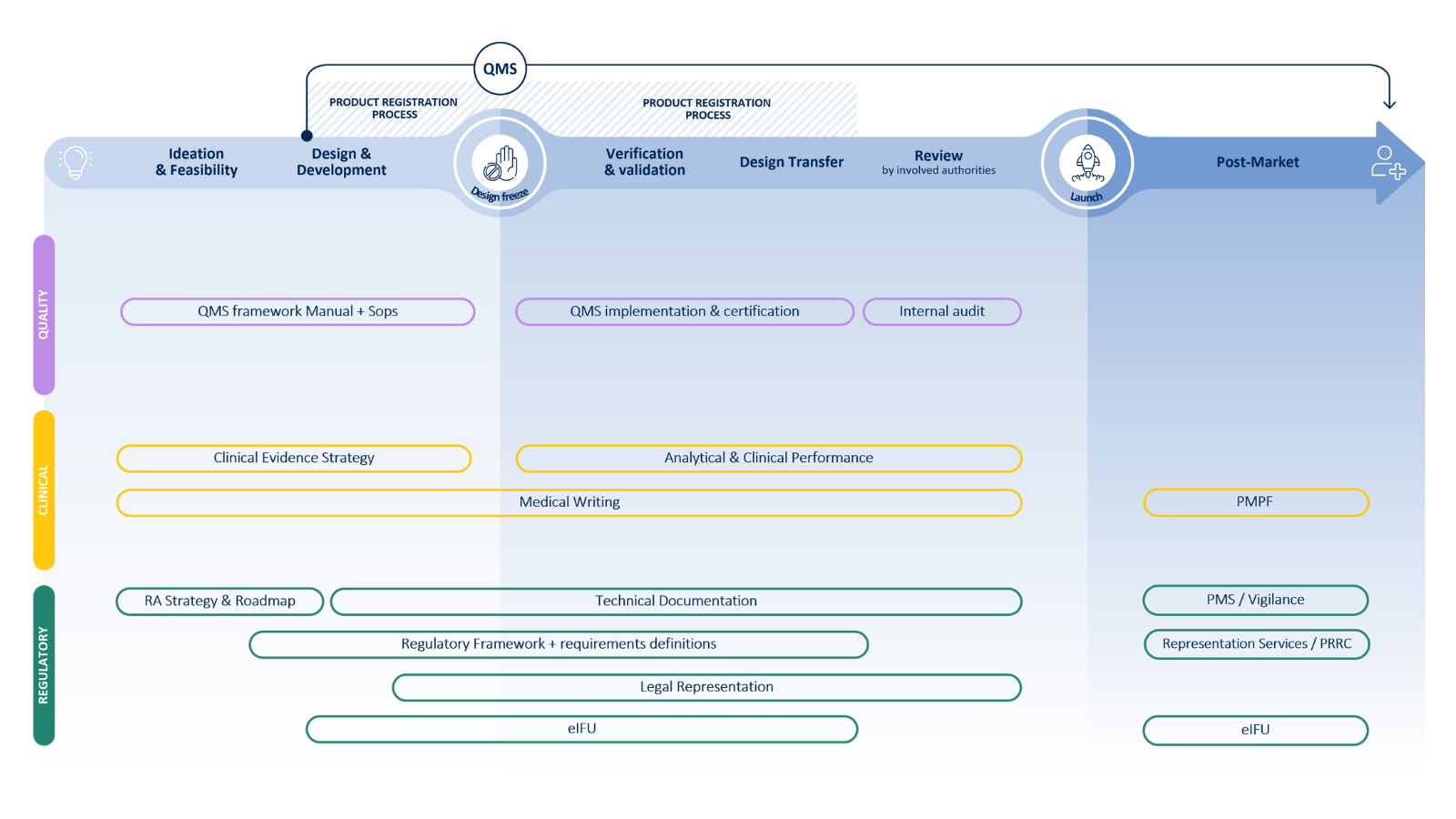
IVDR transition support
We guide IVD manufacturers through the transition from IVDD to IVDR, ensuring compliance and efficiency.
- Quality Assurance: gap assessments, QMS updates, mock audits, and IVDR-compliant process development.
- Regulatory Affairs: strategic regulatory roadmaps, notified body selection, and submission support.
- Technical Documentation: clinical evidence development,
risk management, usability assessments, and IVDR-compliant technical
files.
We cover the full IVD life cycle
From regulatory expertise to quality assurance and clinical evidence, we partner with you to accelerate your journey from idea to patient.





_3604577.png)
_2490489.png)