Companion Diagnostics (CDx) Services
What are companion diagnostics?
TOWARDS REGULATORY AND MARKET ACCESS
Companion Diagnostics (CDx) in the are defined as a device that is essential for the safe and effective use of a corresponding medicinal product.
They are designed to either identify patients who are most likely to benefit from that medication before or during treatment or to identify patients who may be at higher risk of experiencing serious side effects as a result of that medication.
With the implementation of the In Vitro Diagnostic Medical Device Regulation (IVDR), companion diagnostics are now subject to new regulatory requirements that have a significant impact on their development and commercialization.
Since CDx are ideally co-developed with pharmaceutical manufacturers, there are unique challenges involved in bringing together these two distinct regulatory frameworks.
We understand the regulatory complexities involved in the design and development of companion diagnostics, as well as in the development of personalized medicine, which is why we have dedicated regulatory teams with expertise in IVDs and medicinal products that can work together to support your co-development efforts.
This enables us to navigate the challenges involved in bringing companion diagnostics to market, ensuring you can achieve regulatory compliance and commercial success.
What are companion diagnostics?
TOWARDS REGULATORY AND MARKET ACCESS
Companion Diagnostics (CDx) in the EU are defined as a device that is essential for the safe and effective use of a corresponding medicinal product.
They are designed to either identify patients who are most likely to benefit from that medication before or during treatment or to identify patients who may be at higher risk of experiencing serious side effects as a result of that medication.
With the implementation of the In Vitro Diagnostic Medical Device Regulation (IVDR) in the EU, companion diagnostics are now subject to new regulatory requirements that have a significant impact on their development and commercialization.
Since CDx are ideally co-developed with pharmaceutical manufacturers, there are unique challenges involved in bringing together these two distinct regulatory frameworks.
We understand the regulatory complexities involved in the design and development of companion diagnostics, as well as in the development of personalized medicine, which is why we have dedicated regulatory teams with expertise in IVDs and medicinal products that can work together to support your co-development efforts.
This enables us to navigate the challenges involved in bringing companion diagnostics to market, ensuring you can achieve regulatory compliance and commercial success.
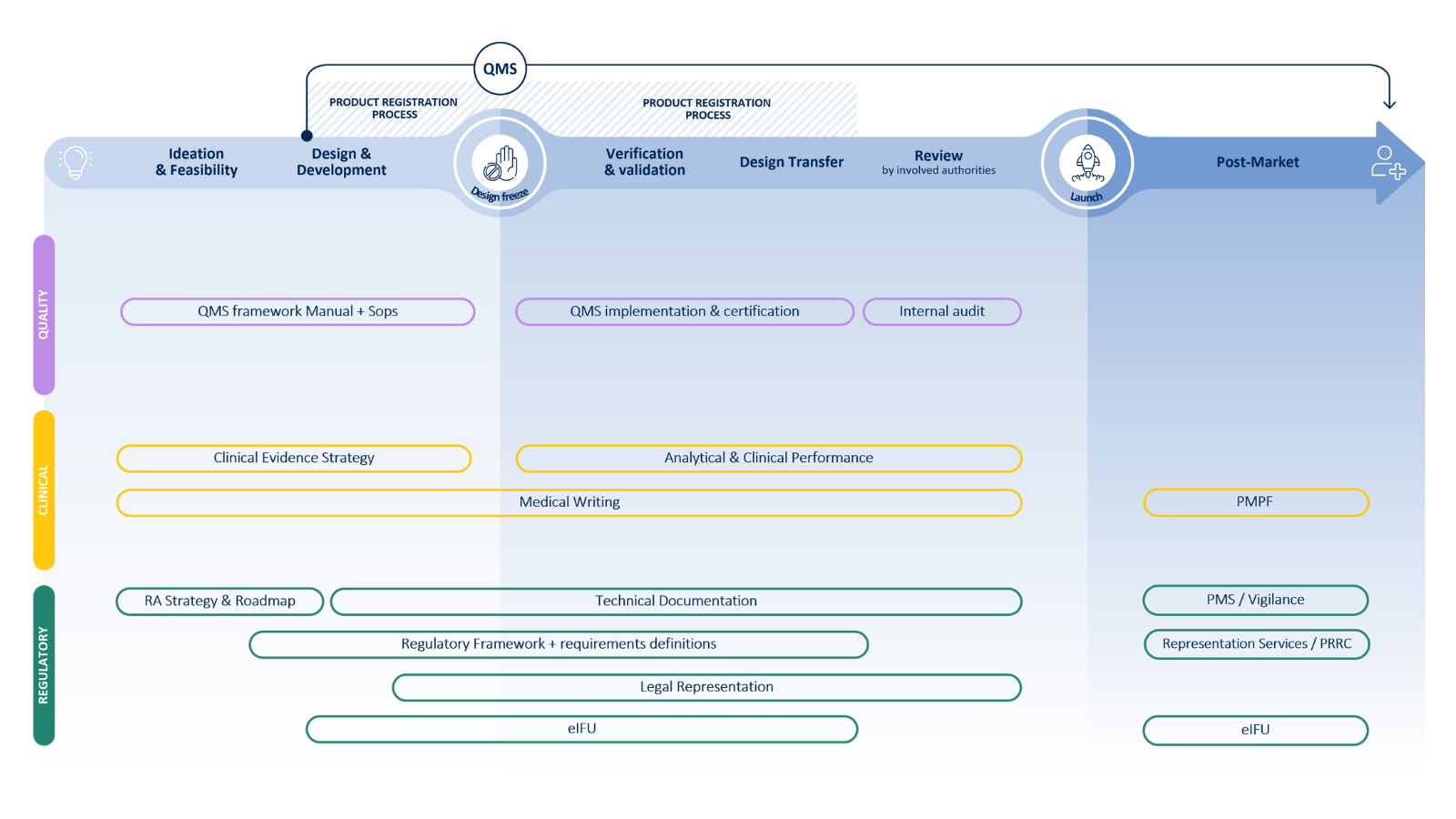
Our companion diagnostics services
Device
Medicinal product

Device
Our services in device development encompass strategic guidance, project management, and comprehensive support throughout all phases of CDx development:
-
Notified body engagement: Assisting with the selection, contracting, and engagement with notified bodies.
-
Submission and review support: Guiding you through the submission and review process of your CDx with the Notified Body.
-
Quality Management System implementation: Helping with the implementation of a compliant Quality Management System (QMS).
-
Clinical performance study design & implementation: Assisting with the design and implementation of your clinical performance study applications.
-
Legal representation: Acting as your legal representative for clinical performance study applications under IVDR Article 58(4).
-
Medical writing and submission package: Reviewing or writing your submission package, along with offering detailed insights on the complex submission strategy in EU member states.
-
Market access strategy: Assisting in developing a market access strategy that encompasses regulatory and reimbursement requirements across various geographies.
-
Health economic assessments and payer engagement: Providing guidance on health economic assessments, pricing, reimbursement negotiations, and engaging with payers.
-
IVDR and CDx Training: Providing on-site or remote training on general or specific aspects of the IVDR.

Medicinal product
Labmark’s expertise extends to the medicinal product aspect of Companion Diagnostic development, where we provide services including:
-
Notified body engagement: Assisting with the selection, contracting, and engagement with Notified Bodies.
-
Submission and review support: Guiding you through the submission and review process of your CDx with the Notified Body.
-
Marketing authorization application: Assisting with the submission and review of marketing authorization applications for medicinal products.
-
Quality Management System implementation: Helping with the implementation of a compliant Quality Management System (QMS).
-
Medical writing: Reviewing, writing, and publishing all eCTD modules required for obtaining marketing authorization.
-
Market access strategy: Assisting in developing a market access strategy that encompasses regulatory and reimbursement requirements across various geographies.
-
Health economic assessments and payer engagement: Providing guidance on health economic assessments, pricing, reimbursement negotiations, and engaging with payers.
Why partner with Labmark?
Our team offers unparalleled knowledge in companion diagnostics, guiding you efficiently through each development phase.
- Strategic Insights for a Competitive
Edge
Accelerate ahead of your competition with our strategic consulting services, tailored to meet the fast-paced demands of the industry. - Comprehensive Solutions for Drug and Device
Development
From initial development to market launch, our experts provide end-to-end solutions for both drugs and devices, ensuring seamless project advancement at every stage.





_3604577.png)
_2490489.png)