
Your trusted CRO for Medical Devices & IVD
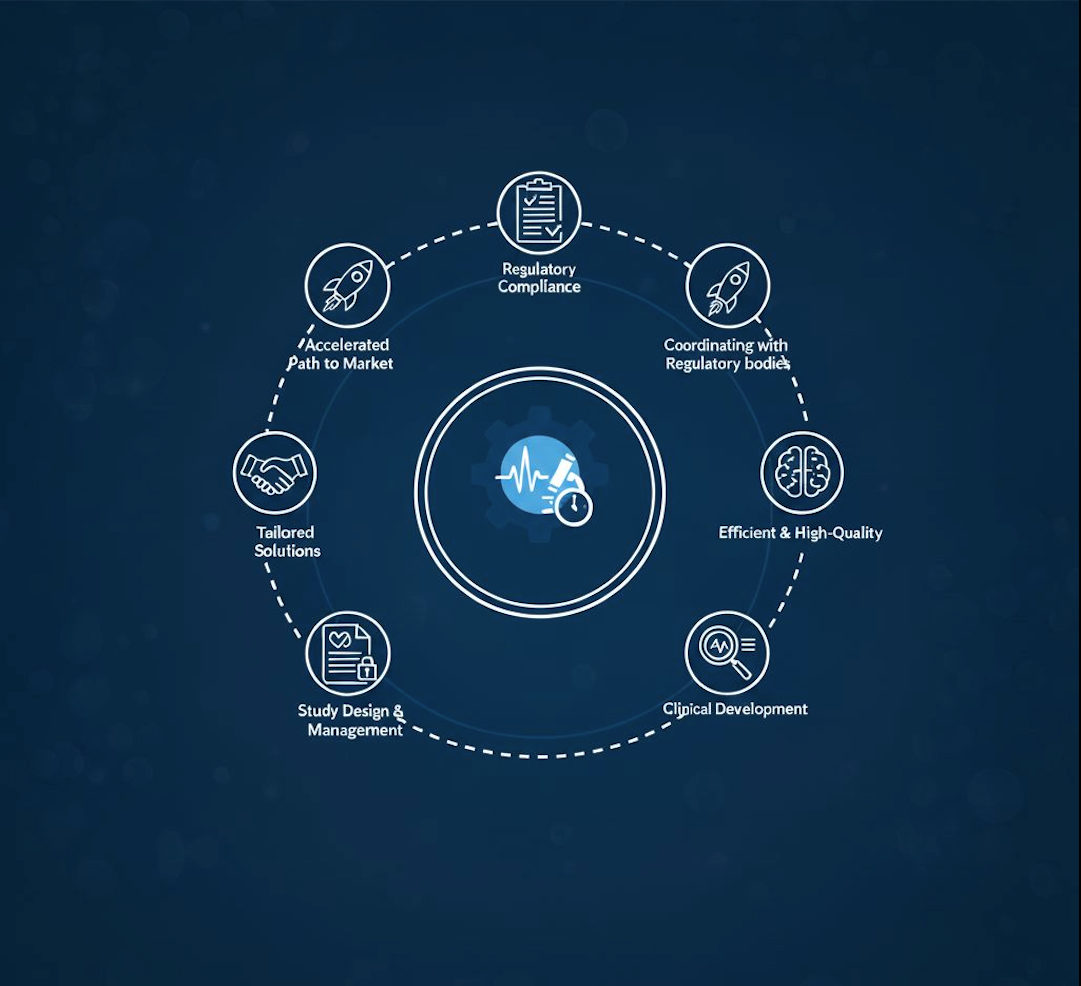
Labmark Clinical is your trusted MD & IVD Contract Research Organization (CRO) for efficient, high-quality clinical development.
With over 10 years of experience, we specialize in designing and managing clinical studies, ensuring regulatory compliance, and accelerating your path to market.
From strategy to execution, we deliver tailored solutions to meet your clinical research needs.
Medtech expertise
We bring a deep understanding of medical device and IVD challenges, specializing in cardiovascular, neurology, orthopedics, and infectious diseases. Unlike pharma-focused CROs, we focus on your industry, providing tailored expertise to support your success.
Trusted partner
With a strong reputation in the MedTech and IVD sectors, we offer reliable support backed by extensive regulatory knowledge, including ICH-GCP, ISO 14155, MDR, IVDR, and EU-CTR. Our commitment fosters trust and enduring partnerships.
Quality at every step
We emphasize quality throughout every stage of development, from planning to execution, reducing risks while maximizing the chances of success for your clinical trials.
Right-sized solutions on budget
Our flexible and scalable approach ensures cost-effective solutions, whether you’re a startup or an established company, helping you stay on track without compromising on quality.
Key therapeutic areas
We have in-depth expertise in the challenges associated with medical devices, with a focus on cardiology, vascular, neurology, and orthopedics.
Unlike CROs that primarily work with pharmaceuticals, we specialize exclusively in medical device clinical trials, offering expert support tailored to your needs.
Cardiovascular Conditions
- Angiography
- Atrial Fibrillation
- Coronary Heart Disease
- Heart Failure
- Coronary Artery Bypass Graft (CABG)
- Aortic Aneurysm (Abdominal, Thoracic)
- Peripheral Artery Disease
- Superficial Femoral Artery (SFA) Disease
- Stroke
Cardiovascular Procedures
- Percutaneous Coronary Intervention (PCI)
- Transcatheter Aortic Valve Replacement (TAVR)
- Transcatheter Mitral Valve Repair (TMVR)
- Neuromodulation
- Angiography
Orthopedic & Vascular Conditions
- Bone Defects
- Infrapopliteal Disease
- Back Pain
- Spine Surgery
- Thoracolumbar Spinal Deformity
- Knee Prosthesis
Neurological & Mental Health Conditions
- Parkinson’s Disease
- Spinal Cord Injury
- Depression
- Brain Aneurysm
Respiratory Conditions
- Asthma
- Chronic Obstructive Pulmonary Disease (COPD)
- Cystic Fibrosis
- Idiopathic Pulmonary Fibrosis
- Sleep Apnea
Oncological Conditions
- Bladder Cancer
- Breast Cancer
- Lymphoma
- Liver Cancer (Hepatocellular Carcinoma, HCC)
- Colorectal Cancer (Metastatic CRC, mCRC)
- Pancreatic Cancer
- Non-Small Cell Lung Cancer (NSCLC)
Infectious Diseases
- COVID-19
- Marburg Virus
- Pneumonia
- HIV/AIDS
- Tuberculosis
- Malaria
- Diarrhea
- Typhoid
CRO services for every stage of your clinical journey
From early feasibility to post-market follow-up, labmarkdx Clinical offers end-to-end Contract Research Organization (CRO) services to accelerate the development and approval of your medical device. Our experts guide you through every step, ensuring compliance, efficiency, and high-quality results.
- Feasibility & Study Design
- Regulatory Submissions
- Medical Writing
- Data Management
- Clinical Monitoring
- Site Management
- Project Management
- Safety Management
- Supply Management
- Core Lab Management
- Quality Assurance & Compliance
- Legal Representation

Trusted by life sciences experts
At Labmark, we take pride in delivering high-quality solutions tailored to our clients’ needs. From clinical trial management to regulatory support, our experts ensure smooth and successful projects.
But don’t just take our word for it, see what our clients have to say!
⭐ 4.5/5 average rating: excellence in clinical support
With a deep understanding of the life sciences industry, labmark Clinical consistently delivers outstanding service. Our clients recognize us for our expertise, proactive approach, and commitment to quality. This is reflected in our 4.5/5 average rating, based on feedback across key areas of collaboration.
Client performance scores
.png?width=240&height=240&name=Customer%20feedback%202025%20(7).png)
.png?width=240&height=240&name=Customer%20feedback%202025%20(8).png)
4.6/5
.png?width=240&height=240&name=Customer%20feedback%202025%20(9).png)
4.6/5
.png?width=240&height=240&name=Customer%20feedback%202025%20(10).png)
4.6/5
.png?width=240&height=240&name=Customer%20feedback%202025%20(11).png)
4.5/5
.png?width=240&height=240&name=Customer%20feedback%202025%20(12).png)
4.6/5
.png?width=240&height=240&name=Customer%20feedback%202025%20(13).png)
4.5/5
Market trends & challenges

Our offerings for Clinical in





Why Labmark?
Labmark combines more than 10 years of experience with flexible, tailored clinical solutions to help you bring products to market efficiently and compliantly.
What sets us apart?
- Proven expertise: Over a decade of clinical experience across industries.
- Global reach: Regulatory expertise across multiple markets.
- Tailored solutions: Flexible support to match your specific project needs.
- Full lifecycle support: From study design to post-market follow-up, we’re with you every step of the way.
Partner with labmark for clinical services that deliver results.





_3604577.png)
_2490489.png)
